DEVA Holding Çerkezköy-II Manufacturing Facility, which has enclosed facilities of 50,270 m² established on an area of 67,551 m2 in Çerkezköy Organized Industrial Zone, at a distance of approximately 110 km from Istanbul, is made up of modern buildings manufacturing solid oncology products, sterile liquid oncology products, animal health products and APIs. There are also R&D, Biotechnology and Central Stability Buildings at the facility.
In 2022, the new Biotechnology Building at the Çerkezköy-II Manufacturing Facility and the Central Stability Building, which will serve all the facilities, commenced its activities after successfully passing the inspections of the Ministry of Health. With the putting in the use of the Biotechnology Building, the Biotechnology R&D team continues its activities in this building. The High Potent Manufacturing Building, whose construction and equipment installations are ongoing, is projected to start its activities in the first quarter of 2023.
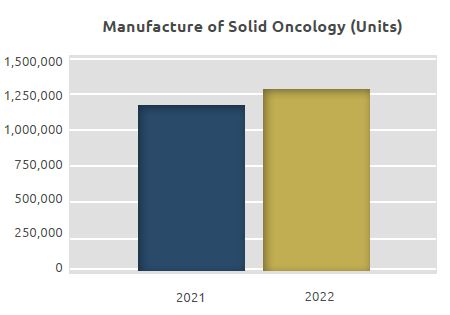
Solid Oncology Manufacturing Unit
Products in the form of tablets and capsules are manufactured in the Solid Oncology Manufacturing Unit.
The total quantity manufactured, which was 1.2 million units in 2021, increased by 9% to 1.3 million units in 2022.

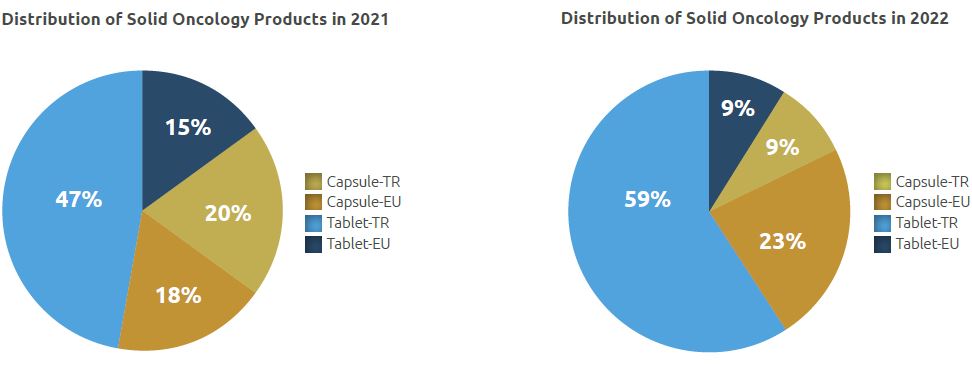
Of a total of 1.3 million units manufactured in 2022, 0.42 million units were manufactured in the form of capsules and 0.87 million units in the form of tablets.
In addition, 0.12 million of 0.87 million units of products manufactured in tablet form and 0.30 million of 0.42 million capsules were manufactured for the European market.
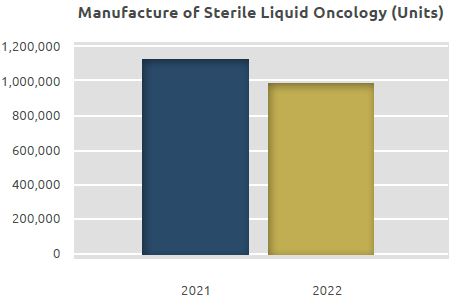
Sterile Liquid Oncology Manufacturing Unit
Products in the form of sterile liquid and sterile lyophilized powder are manufactured in the Sterile Liquid Oncology Manufacturing Unit.
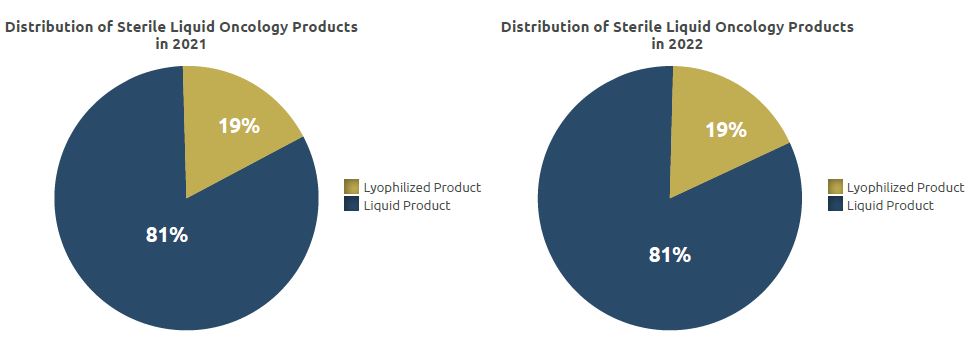
The total quantity manufactured in the unit, which was 1.13 million units in 2021, was realized as 1 million units in 2022.
Of 1 million units of products, 0.81 million units were liquid products and 0.19 million units were lyophilized products.

Non-Sterile (Solid) Animal Health Products Manufacturing Unit
Animal health products are manufactured in the Non-Sterile (Solid) Animal Health Products Manufacturing Unit, 7 of which are in tablet form, 16 in powder form filled in small and large sachets and 1 in powder form filled in bags.
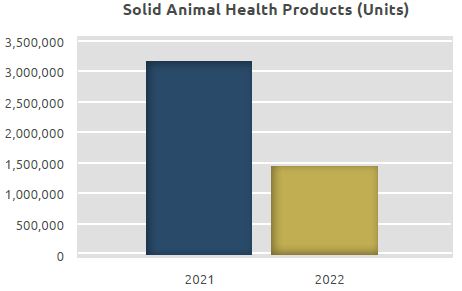
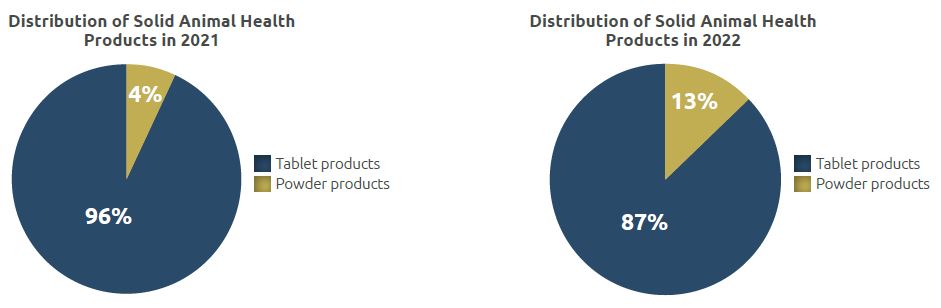
The total quantity manufactured in the unit, which was 3.17 million units in 2021, increased to 1.47 million units in 2022. 1.28 million units of these products are in tablet form while 0.19 million units are in powder form.

API (Active Pharmaceutical Ingredient) Manufacturing Units
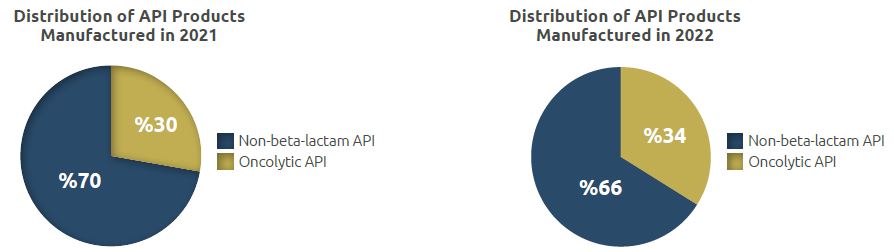
The facility has two physically separate and independent API Manufacturing Units for manufacture of non-betalactam API and oncolytic API.
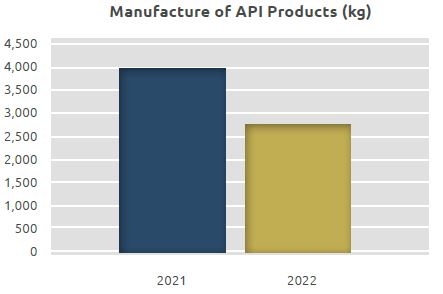
The quantity of the API manufactured was 4,019 kg in 2021, while it was 2,749 kg in 2022.
Of the total API quantity of 2,749 kg manufactured in 2022, 1,828 kg was non-beta-lactam API while 921 kg was oncolytic API.

In API manufacturing, the toxic gases arising from reaction are neutralized before being released to the atmosphere to minimize the environmental effects resulting from manufacturing activities.
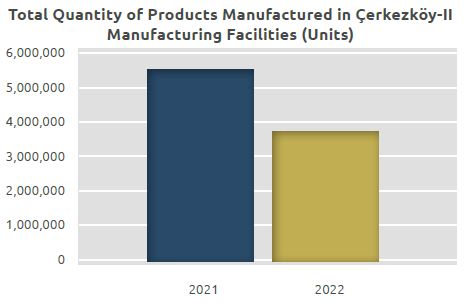
To sum up, the total quantity of finished products manufactured in the Çerkezköy-II Facility of DEVA Holding was 5.5 million units in 2021, while it was 3.8 million units in 2022.
The total capacity utilization rate of the Çerkezköy Manufacturing Facilities is 89.6%.